Phytate
The system that contains higher solute quantities than quantity that allows by its solubility is an unstable situation from the thermodynamic point of view, it is an supersaturated system. The salts in this system sooner or later crystallize to reach equilibrium. The time that a supersaturated system spends for precipitating and cristallize can be from seconds to years and depends on kinetic factors. These kinetic factors are the cause for which indiscriminate crystallizations does not take place in the organism, under normal conditions, even through biological fluids that are supersaturated in some substances.
In fact, there are three
main aspects that should be considered to explain pathological crystallizations:
higher supersaturation above the habitual value (thermodynamic factor), the
presence of heterogeneous nucleus (crystallization promoters), such as macromolecules,
cellular debris, epithelial injures ... (kinetic factor), and/or the deficit
of crystallization inhibitors that are substances that impede or hinder crystals
development (kinetic factor) [1-2]. Under physiologic conditions these factors
are usually equilibrated avoiding crystal formation. However, when a slight
modification takes place in some of them, breaking the equilibrium, indiscriminate
crystallizations appear finally, inducing pathological processes.
It is necessary to point out that under physiologic conditions; supersaturation oscillates between limits with variable values so that when higher supersaturation values are reached some of these crystallization inhibitors are essential in appropriate concentrations. Crystallization inhibitors avoid formation of solid particles to the point that lesser supersaturation values are reached again.
In accordance with the later mentioned, renal calculi formation is a multifactorial problem witch should be considered to be the risk factors related with urine composition and renal morphology. The urine composition factors fundamentally are: excessive supersaturation of the potentially lithogenic substances, deficit of crystallization inhibitors and presence of heterogeneous nucleus. The factors related with renal morphology are mainly: the presence of cavities with low urodynamic efficacy where urine is retained during long periods of time and, also alterations that can take place on the uroepithelium that covers the renal papilla, such as a reduction of the antiadherent layer composed by glycosaminoglycans, epithelial necrosis, etc. The development of renal calculi generally depends on the existence of a number of simultaneous altered factors; nevertheless in most cases only the modification of some factors could avoid the development of new calculus.
|
|
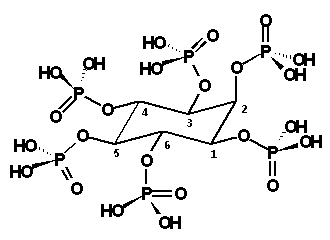
Figure 1. - Structure of the
phytate or myo-inositol 1,2,3,4,5,6-hexakis(dihidrogenophosphate) (IUPAC, 1968). |
Numerous epidemiologic studies show a relationship between calcium renal lithiasis and low phytate intake. Thus, the South African population are constituted mainly by two ethnic groups: European and African origin population. Renal calculi incidence in the European origin population is higher than the African origin population. This difference has been attributed to a different urine composition due to different alimentary habits. The African origin population diet is abundant in whole wheat bread, corn, dry peas, fruits, some vegetables and animal origin foods and, in spite of incorporating new foods, they still follow their old tradition. The great difference among the two groups resides in phosphorous ingestion, since the European origin population consumes most phosphorous as inorganic phosphate form while the African origin population has an important consume in organic phosphorous source as phytate form [3]. Therefore, phytate could diminish the incidence of renal lithiasis in the African origin population, resulting in agreement with the Modlin hypothesis [4]. In another study, phytate intake were compared with one group of calcium oxalate idiopathic renal lithiasis in front of the control group without calcium oxalate renal lithiasis. It was found that phytate intakes were significantly inferior in the calcium oxalate idiopathic lithiasic group [5]. In fact, calcium renal lithiasis incidence in countries with a high consumption of whole meal cereals, richest in phytate, is inferior to the industrialized countries where they consume mainly refined cereals [6]. Studies have also shown that urinary phytate levels are inferior in an calcium oxalate renal lithiasic group compared to a control group of healthy individuals [7]. Recently an epidemiologic dietary study performed through 96.245 individuals demonstrated that phytate intake was associated with a reduced risk of stone formation [8].
The effect of phytate has been studied also on development of calcium oxalate deposits on renal papilla in Wistar rats treated with ethylene glycol (lithiasic agent). Macroscopic and microscopic analysis of kidneys demonstrated that rats to those phytate were given together with the lithiasic agent presented in the renal papilla a lower calcium content and calcifications [18]. Phytate also prevented the appearance of calcifications, in a very effective way, in renal tissue of animals fed with diet lacking of phytate [19].
As already indicated, high sodium phytate intake in hypercalciuric patients was to induce an insoluble calcium complex in the digestive tract, as objective, in order to diminish calcium intestinal absorption. On the other hand, low phytate dietary intake, as calcium-magnesium phytate or phytin, is focused to increase the inhibitory capacity of urine in front of calcium oxalate and calcium phosphate crystallization, hence acting on prevention of calcium renal lithiasis.
Absorption, distribution, excretion and toxicity of phytate
Studies carried
out in experimental animals demonstrated that phytate levels in the organism
were directly related with their oral ingestion, maximum plasmatic levels
were reached with a diet that contained 1 % of phytate in form of sodium salt
or with the same diet containing 0.12 % phytate in form of calcium-magnesium
salt (phytin) from carob bean germ [23].
In fact, a diet
without phytate diminishes the urinary concentration until non detectable
value at 22 days [24]. The addition of higher quantities of phytate to a lacking
phytate diet demonstrated that with a consumption of 20.9 mg/kg of body weight,
a maximum urinary excretion was reached and corresponded to a 2% of the ingested
quantity, and later phytate intake increases did not originate urinary excretion
increases [24].
Total elimination of phytate from the diet during a period of 36 hours produced
urinary level decreases around 50% in human subjects [7]. The study of the
intestinal absorption and urinary excretion of phytate administered orally
to humans demonstrated that phytate urinary levels on two hour urine sample
diminished phytate around 90% and plasmatic levels around 74% after a period
of two weeks of phytate poor diet consumption [25].
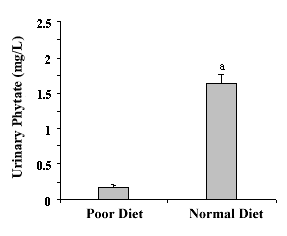 |
Figure
2.
- Urinary Phytate with Urinary phytate following a phytate poor diet and
after follow a normal diet in phytate. The values are expressed as mean.
The values are expressed as the mean± SEM of seven volunteers.
a p<0.05 vs. poor diet [25]. |
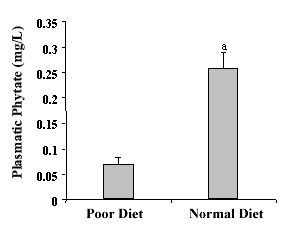 |
Figure 3. - Plasmatic Phytate with Urinary phytate following a phytate poor diet and after follow a normal diet in phytate. The values are expressed as mean. The values are expressed as the mean± SEM of seven volunteers . a p<0.05 vs. poor diet [25. |
It can be deduced
that urinary and plasmatic levels of phytate depend basically on their diet
intake, and the human organism can not synthesise enough endogenous phytate
de novo from inositol to maintain normal phytate levels. These studies
evidenced that phytate was absorbed quickly with a maximum of plasmatic absorption
at 4 hours after intake; although only a low percentage of orally administered
dose was absorbed [25].
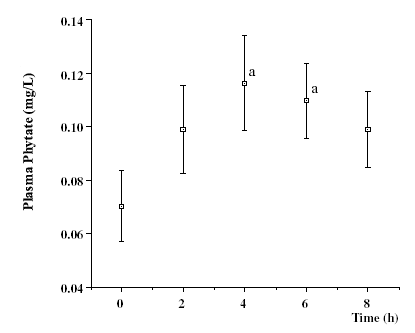 |
Figure.
4. - The kinetic of phytate after ingestion of a single dose of 1400 mg
sodium phytate following a IP6-poor diet. Values are mean ± SE
of 7 subjects. Student t-test was used to determine statistic significance
between means. a p < 0.05 vs 0 hours-time. (1.00 mg/L = 1.52 µmol/L). [15]. |
Excretion values of urinary phytate did not show significant differences after administration of three different salts doses to subjects following a low phytate diet. Salts doses were: 400 mg of phytin (308 mg phytate), 3 200 mg of phytin (2 470 mg phytate) and 1 400 mg of sodium phytate (1000 mg phytate)l [25]. Six fold increase of the dose did not produce any considerable increment of urinary phytate excretion. These results agree totally with studies carried out with experimentation animals, demonstrating that with a low phytate dose maximum urinary excretions are reached. On the other hand, these results also demonstrate that the quantities of phytate excreted in the urine are no modified by the type of salt given..
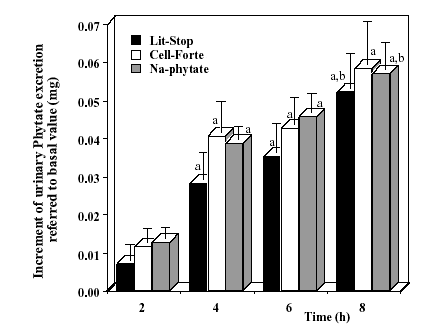 |
Figures 5. - Increase of urinary phytate
excretion every two hours referred to the first urine sample, after the
one dose ingestion of 400 mg of phytin, 3,200
mg of phytin and 1,400 mg of sodium phytate, after a continuing
period of two weeks with a phytate poor diet. The values are expressed
as means ± SEM of seven volunteers. a p <0.05 vs. 2 hours and
b p <0.05 vs. 4 hours [15]. |
When the same subjects, that are following a low phytate diet, went onto
normal phytate diet, a rise of plasma phytate was produced at 15 days:
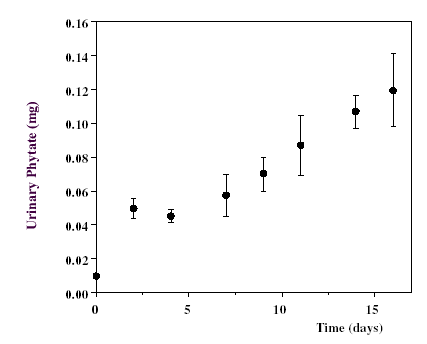 |
Figure 6. - Increasing levels of phytate
in 2 h-urine of healthy volunteers on phytate normal diet following a
two weeks period of phytate poor diet. (1.00 mg = 1.52 mol).The values are expressed as the mean ± SEM of seven volunteers [15]. |
However, the
values reached at 16 day had still not stabilized [25]. Plasmatic concentration
phytate values, as can be appreciated, were directly related with their urinary
excretion in a urinary sample of two hours obtained after the blood sample
collection [25].
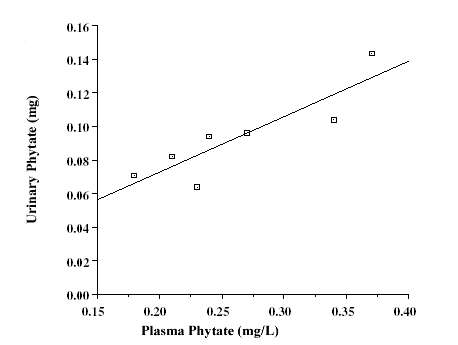 |
Figure 7. Correlation between phytate plasma levels and phytate urinary excretion (in a two hours urinary sample) previous to blood sample collection in seven volunteers with a normal diet [15]. |
The urinary phytate
concentration can be a marker of the organism phytate status. In this aspect
we should consider that as human diet do not follow a regular phytate consumption
like experimental animals that are feed on the same diet the urinary levels
in experimental animals increase and stabilize more quickly.
Other studies
demonstrated that phytate is distributed in all organs and fluids of experimental
animals. Phytate concentration determination in experimental animals fed with
synthetic diet where phytate was added as sodium salt, evidenced that phytate
concentrations varied according to characteristics of tissue, from 0.2 mg/L
in plasma to 20 mg/g in brain, while when the same diet lacking in phytate
was given phytate values decreased to 0.02 mg/L in plasma and to 0.9 mg/g
in brain [26].
Table I. Phytate distribution in organs and corporal fluids of rats
fed with 1% of sodium phytate added to the diet with and with a diet without
phytate. Phytate tissue concentration is expressed as mg by dry tissue.
|
WHITH phytate
|
WHITH OUT phytate
|
|
| Urine(mg/l) |
2.51
|
non detectable
|
| Plasma (mg/l) |
0.19
|
0.02
|
| Kidney (mg/g) |
1.44
|
0.04
|
| Liver (mg/g) |
2.26
|
non detectable
|
| Bone (femur) (mg/g) |
1.40
|
non detectable
|
| Brain (mg/g) |
20.16
|
0.90
|
Finally we should point out those acute toxicity studies of phytate as sodium phytate has been realised. The DL50 in rats and mice, administered via oral, was 400 and 2.750 mg/kg respectively [27-28].
BIBLIOGRAFIA
1. Grases F, Costa-Bauzá A. Phytate is a powerful agent for preventing calcifications in biological fluids: usefulness in renal lithiasis treatment. Anticancer Res 1999; 19: 3717-3722.
2. Grases F, Costa-Bauzá A, Königsberger E, Königsberger L-C. Kinetic versus thermodynamic factors in calcium renal lithiasis. Int Urol Nephrol 2000; 32: 19-27.
3. Modlin M.
The aetiology of renal stones: a new concept rising from studies on a stone
free population. Ann R Coll Surg England 1967; 40: 155-159.
4. Modlin M. Urinary phosphorylated inositols and renal stone. Lancet 1980; 2: 1113-1114.
5. Griffith HM, Oshea B, Maguire M, Koegh B, Kevany JP. A case-control study of dietary intake of renal stone patiens. II. Urine biochemistry and stone analysis. Urol Res 1986; 14: 75-82.
6. Andersen DA. Historical and geographical differences in the pattern of incidence of urinary stones considered in relation to possible aetiological factors. En: Renal Stone Research Symp Pags. 7-16. Hodgkinson A y Nordin BEC Eds. Churchill. 1969; Londres.
7. Grases F, March JG, Prieto RM, Simonet BM, Costa-Bauzá A, García-Raja A, Conte A. Urinary phytate in calcium oxalate stone formers and healthy people. Dietary effects on phytate excretion. Scand J Urol Nephrol 2000; 34: 162-164.
8. Grases F, Kroupa M, Costa-Bauzá A. Studies on calcium oxalate monohydrate crystallization. Influence of inhibitors. Urol Res 1994; 22: 39-43.
9. Grases F, Costa-Bauzá A. Potentiometric study of the nucleation of calcium oxalate in presence of several additives. Clin Chem Enzym Comms 1991; 3: 319-328.
10. Grases F, Ramis M, Costa-Bauzá A. Effects of phytate and pyrophosphate on brushite and hydroxyapatite crystallization. Comparison with the action of other polyphosphates. Urol Res 2000; 28: 136-140.
11. Grases F, March P. A study about some phosphate derivatives as inhibitors of calcium oxalate crystal growth. J Crystal Growth 1989; 96: 993-995
12. Grases F, García-Ferragut L, Costa-Bauzá A. Study of the early stages of renal stone formation. Experimental model using urothelium of pig urinary bladder. Urol Res 1996; 24: 305-311.
13. Grases F, García-Ferragut L,Costa-Bauzá A, March JG. Study of the effects of different substances on the early stages of papillary stone formation. Nephron 1996; 73: 561-568.
14. Grases F, Costa-Bauzá A, March JG. Artificial simulation of the early stages of renal stone formation. Brit J Urol 1994; 74: 298-301.
15. Grases F, García-Ferragut L, Costa-Bauzá A. Development of calcium oxalate crystals on urothelium: effect of free radicals. Nephron 1998; 78: 296-301.
16. Grases F, Llobera A. Experimental model to study sedimentary kidney stones. Micron 1998; 29: 105-111.
17. Finlayson B. Physicochemical aspects of urolithiasis. Kidney Int 1978; 13: 344-360.
18. Grases F, García-Gonzalez R, Torres JJ, Llobera A. The effects of phytic acid on renal stone formation in rats. Scand J Urol Nephrol 1998; 32: 261-265.
19. Grases F., Prieto R.M., Simonet B.M., March J.G. (2000) Phytate prevents tissue calcifications in female rats. BioFactors 11: 171-177
20. Henneman PH, Benedict PH, Forbes AP, Dudley HR. Idiopathic hypercalciuria. N Eng J Med 1958; 17: 802-807.
21. Grases F, García-Ferragut L, Costa-Bauzá A. A new procedure to evaluate the inhibitory capacity of calcium oxalate crystallization in whole urine. Int Urol Nephrol 1995; 27: 653-661.
22. Conte A,
Pizá P, García-Raja A, Grases F, Costa-Bauzá A, Prieto
RM. Urinary lithogen risk test: usefulness in the evaluation of renal lithiasis
treatment using crystallization inhibitors (citrate and phytate). Arch Esp
Urol 1999; 52: 305-310.
23. Grases F, Simonet BM, Prieto RM, March JG. Dietary phytate and mineral bioavailibility. J Trace Elem Med Biol 2001; 15: 221-228.
24. Grases F, Simonet BM, March JG, Prieto RM. Inositol hexakisphosphate in urine: the relationship between oral intake and urinary excretion. BJU Int 2000; 85: 138-142.
25. Grases F, Simonet BM, Vucenik I, Prieto RM, Costa-Bauzá A, March JG, Shamsuddin AM. Absorption and excretion of orally administered inositol hexaphosphate (IP6 or phytate) in humans. BioFactors 2001; 15: 53-61.
26. Grases F, Simonet BM, Prieto RM, March JG. Phytate levels in diverse rat tissues: influence of dietary phytate. Brit J Nutr 2001; 86: 1-8.
27. Fujitani T, Yoneyama M, Kabashima J, Hosokawa N, Ichikawa H. Acute toxicity of phytic acid and sodium phytate in mice. Kenkyu Nenpo-Tokyo-toristu Eisei Kenkyusho 1987; 38: 368-370.
28. Ichikawa H, Ohishi S, Takahashi O, Kobayashi H, Yuzawa K, Hosokawa N, Hashimoto T. Acute oral toxicities of phytic acid and sodium phytate in rats. Kenkyu Nenpo-Tokyo-toristu Eisei Kenkyusho 1987; 38: 371-376.